Tree of the month: Methyl-accepting chemotaxis proteins in Escherichia coli and bacterial gradient sensing
 Let’s use our imagination for a moment. We would like you to imagine that we are in a far future, in a time when humanity has the ability to freely explore the vast distances between the stars. Let’s imagine we are part of one of these brave gangs in one of these galactic vessels. Now let’s imagine that our spaceship is lost in the middle of a nebula, a giantic gas and dust cloud that spans as far as your eyes can see, and probably several light-years beyond. The cloud density blocks the instruments, rendering impossible to navigate guiding us by the position of the stars. Fortunately, we can gather all we may need for survival from the nebula itself. There is oxygen for us and our crew, there is hydrogen to fuel the motors, and there is even carbon and nitrogen that can be used by the ship’s systems to synthesize the disgusting but nutritive ooze we eat every day. As long as everyone’s mind can endure the isolation, all of us will be fine. However, our space odyssey cannot be complete without a proper danger, and in this case we know that the nebula dust is dangerous for the filters, and that some regions contain particles big enough for damaging the ship’s hull. Since the composition of the gas cloud is heterogeneous, we should be able to move wisely, searching the regions with higher density of resources while avoiding the space dust. But how can we know the right trajectory if the instruments cannot see through the infiniteness of the cloud we are trapped in?
Let’s use our imagination for a moment. We would like you to imagine that we are in a far future, in a time when humanity has the ability to freely explore the vast distances between the stars. Let’s imagine we are part of one of these brave gangs in one of these galactic vessels. Now let’s imagine that our spaceship is lost in the middle of a nebula, a giantic gas and dust cloud that spans as far as your eyes can see, and probably several light-years beyond. The cloud density blocks the instruments, rendering impossible to navigate guiding us by the position of the stars. Fortunately, we can gather all we may need for survival from the nebula itself. There is oxygen for us and our crew, there is hydrogen to fuel the motors, and there is even carbon and nitrogen that can be used by the ship’s systems to synthesize the disgusting but nutritive ooze we eat every day. As long as everyone’s mind can endure the isolation, all of us will be fine. However, our space odyssey cannot be complete without a proper danger, and in this case we know that the nebula dust is dangerous for the filters, and that some regions contain particles big enough for damaging the ship’s hull. Since the composition of the gas cloud is heterogeneous, we should be able to move wisely, searching the regions with higher density of resources while avoiding the space dust. But how can we know the right trajectory if the instruments cannot see through the infiniteness of the cloud we are trapped in?For us, brave space wanderers on board of a futuristic spacecraft, finding our path in the middle of the nebula is a big adventure. However, for bacteria and other microbes this situation is not science-fiction. Microorganisms have to swim across gradients of nutrients and damaging agents, and decide their route based only in sensory input from their immediate surroundings. Thus, if we discover how they do it we may be able to solve our little crisis in the nebula.
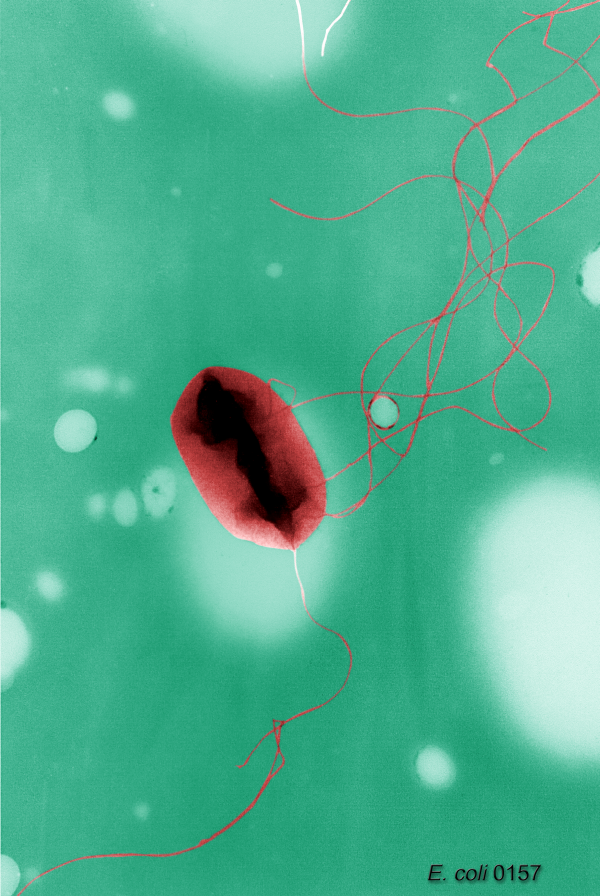 The model bacteria Escherichia coli propels itself thanks to a mane of rigid spring-shaped spinning flagella. Most of the time the bacteria swims in a straight line, with all the flagella aligned in the same axis and spinning in the same sense. The bacteria can change the direction of its movement, but since it doesn’t have any kind of reference it does it randomly turning to a new direction in a process called “tumbling”. Tumbling requires that some of the flagella modify their spinning sense and alignment with the rest for a brief moment, which consequently modifies the orientation of the cell and the direction of swimming in an uncontrolled way. Under homogeneous conditions, tumbling happens with a given frequency that produces a random trajectory for the cell. However, in the presence of a gradient of any attractive substance tumbling frequency decreases if the cell detects an increase in the substance concentration and vice versa. On this way the bacteria keeps trajectories that lead to favorable conditions while rapidly correcting those that are worse than the previous one. While it is a beautiful solution to the problem, it needs the bacteria to at least keep track of the concentration of the different substances in a immediate past, so it can compare it with the current situation and take the decision of increasing or decreasing the frequency of tumbling. Putting it simply, it implies that some form of memory exists in bacteria.
The model bacteria Escherichia coli propels itself thanks to a mane of rigid spring-shaped spinning flagella. Most of the time the bacteria swims in a straight line, with all the flagella aligned in the same axis and spinning in the same sense. The bacteria can change the direction of its movement, but since it doesn’t have any kind of reference it does it randomly turning to a new direction in a process called “tumbling”. Tumbling requires that some of the flagella modify their spinning sense and alignment with the rest for a brief moment, which consequently modifies the orientation of the cell and the direction of swimming in an uncontrolled way. Under homogeneous conditions, tumbling happens with a given frequency that produces a random trajectory for the cell. However, in the presence of a gradient of any attractive substance tumbling frequency decreases if the cell detects an increase in the substance concentration and vice versa. On this way the bacteria keeps trajectories that lead to favorable conditions while rapidly correcting those that are worse than the previous one. While it is a beautiful solution to the problem, it needs the bacteria to at least keep track of the concentration of the different substances in a immediate past, so it can compare it with the current situation and take the decision of increasing or decreasing the frequency of tumbling. Putting it simply, it implies that some form of memory exists in bacteria.
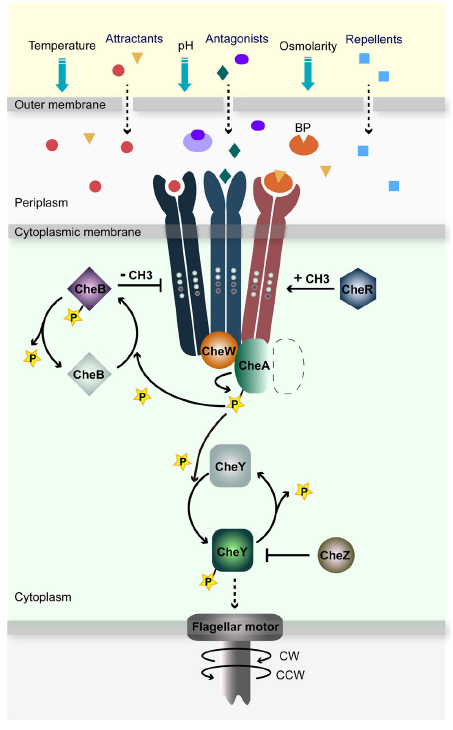 Bacterial flagellum have fascinated scientist for decades, virtue of its exquisite proteinic gears. Unlike eukaryotic flagella that moves using a contraction mechanism, bacterial flagella use a spinning motor mechanism that is loosely related with respiratory ATP synthases. In bacteria, the motor usually rotates in a counterclockwise sense, but it switches its spinning sense stochastically with a given frequency. The switching frequency is controlled by a signaling pathway whose components are labeled as “che” proteins, fror their involvement in chemotaxis. These proteins transduce the signal from an array of membrane receptors labelled as methyl-accepting chemotaxis proteins (MCP) that are the key of the bacterial memory we previously predicted. E. coli K12 has five of these receptors that are able to respond to different substances. Three dimers of different paralogous receptors bind to cheW, forming a complex of sensory proteins. The complex is associated with cheA, a kinase with autophosphorylation activity protein. Ligand binding to the receptor frees cheA which induces clockwise turn in the motor through transient phosphorylation of cheY. All the signalling pathway for the different receptors converge in cheY, making it the equivalent of the bacterial steering wheel. The structural changes in the receptor are mediated by cheR and cheB, a methylase and a methyl esterase that add or remove methyl groups in specific glutamate residues of the MCP. cheB and cheR activity is regulated by the conformation of the receptor. Since the methylation level affects the sensibility of the receptor and given the delay in the methylation-demethylation response, cheA activity is dependent of the previous state of the system. A schematic representation of the complete pathway can be seen in the figure extracted from Bi & Lai (2015).
Bacterial flagellum have fascinated scientist for decades, virtue of its exquisite proteinic gears. Unlike eukaryotic flagella that moves using a contraction mechanism, bacterial flagella use a spinning motor mechanism that is loosely related with respiratory ATP synthases. In bacteria, the motor usually rotates in a counterclockwise sense, but it switches its spinning sense stochastically with a given frequency. The switching frequency is controlled by a signaling pathway whose components are labeled as “che” proteins, fror their involvement in chemotaxis. These proteins transduce the signal from an array of membrane receptors labelled as methyl-accepting chemotaxis proteins (MCP) that are the key of the bacterial memory we previously predicted. E. coli K12 has five of these receptors that are able to respond to different substances. Three dimers of different paralogous receptors bind to cheW, forming a complex of sensory proteins. The complex is associated with cheA, a kinase with autophosphorylation activity protein. Ligand binding to the receptor frees cheA which induces clockwise turn in the motor through transient phosphorylation of cheY. All the signalling pathway for the different receptors converge in cheY, making it the equivalent of the bacterial steering wheel. The structural changes in the receptor are mediated by cheR and cheB, a methylase and a methyl esterase that add or remove methyl groups in specific glutamate residues of the MCP. cheB and cheR activity is regulated by the conformation of the receptor. Since the methylation level affects the sensibility of the receptor and given the delay in the methylation-demethylation response, cheA activity is dependent of the previous state of the system. A schematic representation of the complete pathway can be seen in the figure extracted from Bi & Lai (2015).
As we can see in our tree of the month, all five E. coli MCP are related and generated by gene duplication prior to the divergence of Escherichia and Salmonella. In other species the number is highly variable (just look at Pseudomonas syringae in the tree!), due to gene loss, duplication, domain rearrangements and horizontal gene transfer events. Even within Escherichia coli strains drastical arrangements have been identified, which related with certain specific phenotypes as seen in Borziak et al (2013). This dynamism is expected given the great diversity of bacterial ecologies but highly contrasts with the high conservation of the overall flagellar machinery across the bacterial (and also archaeal!) tree of life.
References:
http://www.ncbi.nlm.nih.gov/pubmed/25374297
http://www.ncbi.nlm.nih.gov/pubmed/23749975
