Tree of the month: Salinibacter ruber archaeal-like bacteriorhodopsin
 It is already december and, for many people in the north hemisphere, that means white and snowy landscapes. When the cold arrives and the snowflakes begin to fly in the air like fragile butterflies, many people will grab their coats and go to play with the spongy and cold substance fallen from the sky. Those that do not like the cold will probably cuddle in front of the stove or the chimney, perhaps enjoying a hot cup of chocolate. Sure, some will be audaciously defending their beloved summer but even them will agree in one thing: you cannot build a snowman out of sunrays. But as fun as it may be, snow can also be a hindrance. A big snowstorm can easily block roads and even trap people inside buildings, while even a little amount can make driving a dangerous sport.
It is already december and, for many people in the north hemisphere, that means white and snowy landscapes. When the cold arrives and the snowflakes begin to fly in the air like fragile butterflies, many people will grab their coats and go to play with the spongy and cold substance fallen from the sky. Those that do not like the cold will probably cuddle in front of the stove or the chimney, perhaps enjoying a hot cup of chocolate. Sure, some will be audaciously defending their beloved summer but even them will agree in one thing: you cannot build a snowman out of sunrays. But as fun as it may be, snow can also be a hindrance. A big snowstorm can easily block roads and even trap people inside buildings, while even a little amount can make driving a dangerous sport.

 Eppur si muove. A simple microscopic preparation of a water sample from a salt pond, a place where seawater is placed to be evaporated and produce salt, will reveal the presence of living organisms. And while diversity and abundance of life forms are reduced as the salt concentration raises, it never disappears completely. Most of these organisms are microscopic archaea, members of the fascinating (although sometimes forgotten) third domain of life. Archaea can be found near everywhere, but are quite adept at adapting to extreme environments, where they usually form the big bulk of the microbial community. While they look much like bacteria, archaea have certain biochemical peculiarities and a fascinating evolutionary history. Hiperhalophylic archaea are incredible organisms that accumulate salt at high concentrations inside themselves in order to be able to scavenge water from their surrounding. As an example, the picture on the left shows a microscopic fluorescent preparation of Haloquadratum walsbyi. H. walsbyi is highly abundant in the saltiest parts of salt ponds around the world, but during decades it was hidden in plain sight before the microbiologist community. Due to its square shape everyone thought that they were just salt crystals!
Eppur si muove. A simple microscopic preparation of a water sample from a salt pond, a place where seawater is placed to be evaporated and produce salt, will reveal the presence of living organisms. And while diversity and abundance of life forms are reduced as the salt concentration raises, it never disappears completely. Most of these organisms are microscopic archaea, members of the fascinating (although sometimes forgotten) third domain of life. Archaea can be found near everywhere, but are quite adept at adapting to extreme environments, where they usually form the big bulk of the microbial community. While they look much like bacteria, archaea have certain biochemical peculiarities and a fascinating evolutionary history. Hiperhalophylic archaea are incredible organisms that accumulate salt at high concentrations inside themselves in order to be able to scavenge water from their surrounding. As an example, the picture on the left shows a microscopic fluorescent preparation of Haloquadratum walsbyi. H. walsbyi is highly abundant in the saltiest parts of salt ponds around the world, but during decades it was hidden in plain sight before the microbiologist community. Due to its square shape everyone thought that they were just salt crystals!
However, while archaea are the protagonists in those environments, they are not the only inhabitants. Some bacteria have learned to use the same tricks that use the archaea and can live in the same extreme environments. Salinibacter ruber, discovered in 2002 in Spain, is an example of this, as it is able to accumulate salt in its cytoplasm just like halophilic archaea do. This mimicry is by no means coincidence, as many of the genes that are used by the archaea have been found in the genome of Salinibacter.
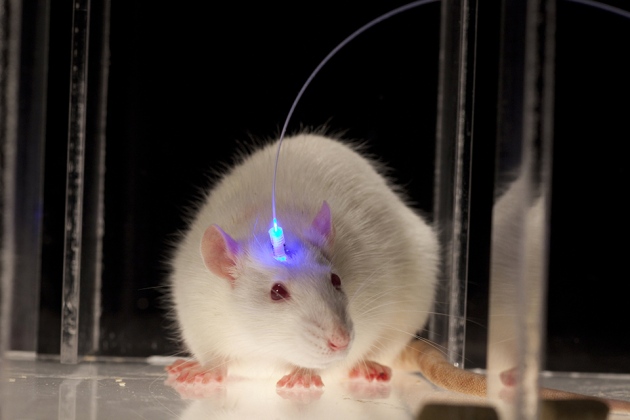 One of these gifts, that we are going to use to exemplify the rest, is the bacteriorhodopsin. This gene encodes a transmembrane protein that uses the light-induced conformational change of a retinol molecule to pump protons to the periplasmic reticulum. Proton gradients are used by bacteria for many ends, such as ATP-production, flagellar motion or active intake of food. Thus, this light-dependent pump ican be considered as the simplest known form of photosynthesis. There are many other light-dependent ion pumps that have been identified in all these hyperhalophilic organisms, some of them being able to pump many other ions, such as chloride or sodium. By expressing these proteins on a cell scientists can use a light source, such as a laser, to induce membrane depolarization. This technology, known as optogenetics, is being widely applied to neurosciences, where the ability to depolarize neurones with a laser is a valuable asset to perform experiments, offering high control and precision with little invasiveness even in living animals.
One of these gifts, that we are going to use to exemplify the rest, is the bacteriorhodopsin. This gene encodes a transmembrane protein that uses the light-induced conformational change of a retinol molecule to pump protons to the periplasmic reticulum. Proton gradients are used by bacteria for many ends, such as ATP-production, flagellar motion or active intake of food. Thus, this light-dependent pump ican be considered as the simplest known form of photosynthesis. There are many other light-dependent ion pumps that have been identified in all these hyperhalophilic organisms, some of them being able to pump many other ions, such as chloride or sodium. By expressing these proteins on a cell scientists can use a light source, such as a laser, to induce membrane depolarization. This technology, known as optogenetics, is being widely applied to neurosciences, where the ability to depolarize neurones with a laser is a valuable asset to perform experiments, offering high control and precision with little invasiveness even in living animals.
Horizontal gene transfer is incredibly common in extremophilic prokaryotes. However, while this phenomenon allow the microbes to adapt to new and harsh environments, these presents are a great challenge for the reconstruction of species phylogenies. As we can observe in our tree of the month, Salinibacter ruber bacteriorhodopsin is embedded in a group consisting exclusively of hiperhalophylic archaeal sequences. While other bacteria do also have bacteriorhodopsins, the gene from Salinibacter is clearly of archaeal origin and thus we observe it placed in the tree as if it were another member of the family Halobacteriaceae. What a festive scene! Many other stories await you in the phylome 20, Salinibacter ruber M8 in the context of other 347 bacterial and archaeal species!
References:
http://www.ncbi.nlm.nih.gov/pubmed/23373661
http://www.ncbi.nlm.nih.gov/pubmed/22623305
http://www.ncbi.nlm.nih.gov/pubmed/26442282
http://www.ncbi.nlm.nih.gov/pubmed/26308982
http://www.ncbi.nlm.nih.gov/pubmed/23831552
Pictures:
Snowman picture: "Snowman on frozen lake" by Petritap - Own work. Licensed under CC BY-SA 3.0 via Commons - https://commons.wikimedia.org/wiki/File:Snowman_on_frozen_lake.jpg#/media/File:Snowman_on_frozen_lake.jpg
Mouse picture by John B. Carnett/Popular Science/Getty
